| Catalog Number | Size | Price | Quantity |
|---|---|---|---|
| FLD0601 | 0.5 mL | ¥1600.0 | |
| FLD0601-1 | 1 mL | ¥2800.0 |
Thiazole Green I is a highly sensitive, low-toxicity, and stable green fluorescent dye designed for the detection of double-stranded DNA (dsDNA) in agarose and polyacrylamide gels. Upon binding to dsDNA, it exhibits a marked increase in fluorescence intensity, offering significantly higher sensitivity than traditional ethidium bromide (EtBr) and enabling the detection of low-abundance DNA at the picogram level. With strong affinity for dsDNA and exceptionally low background fluorescence, it delivers high signal-to-noise ratios, facilitating clear band visualization, image analysis, and DNA recovery assessment.
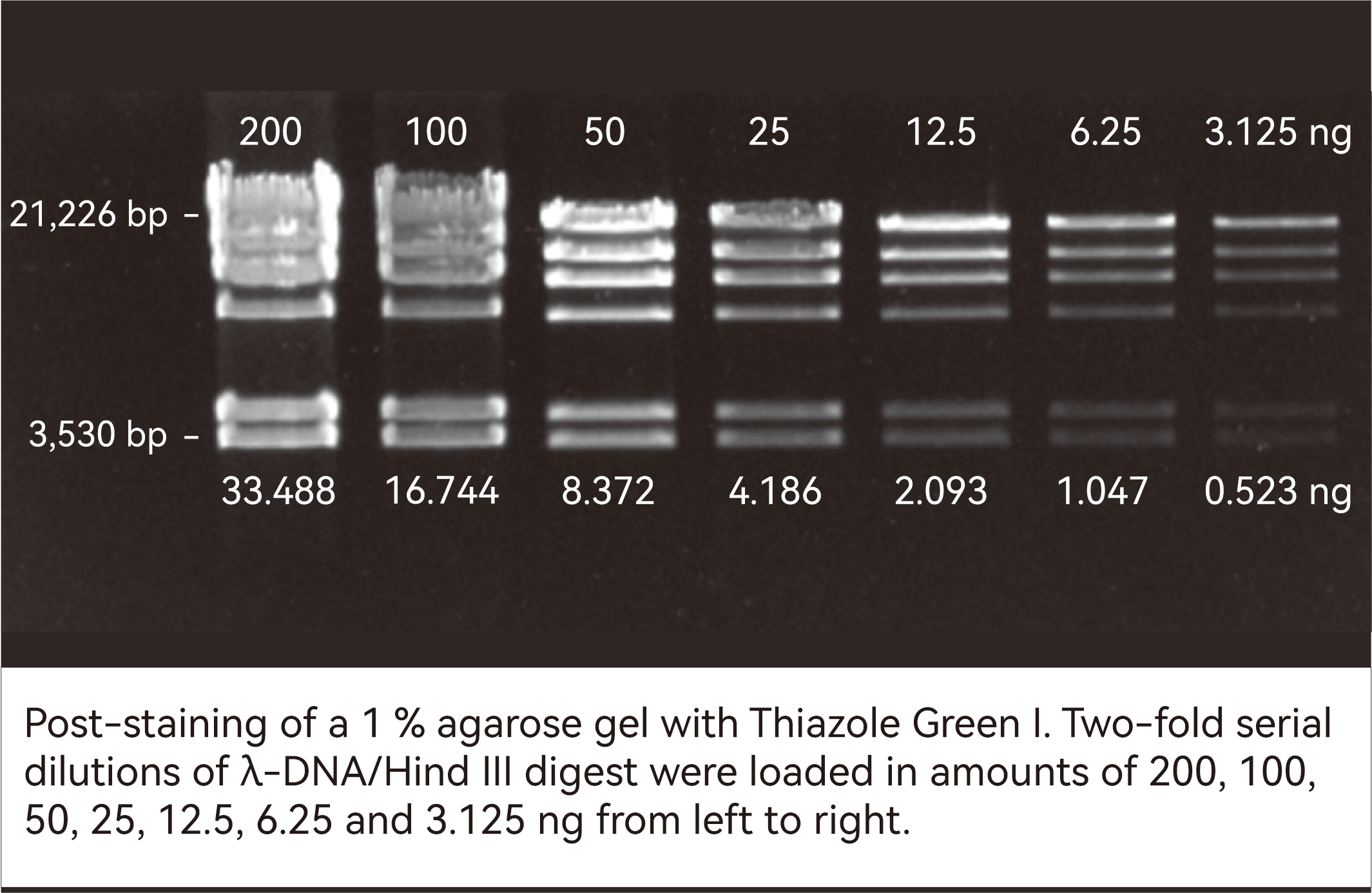
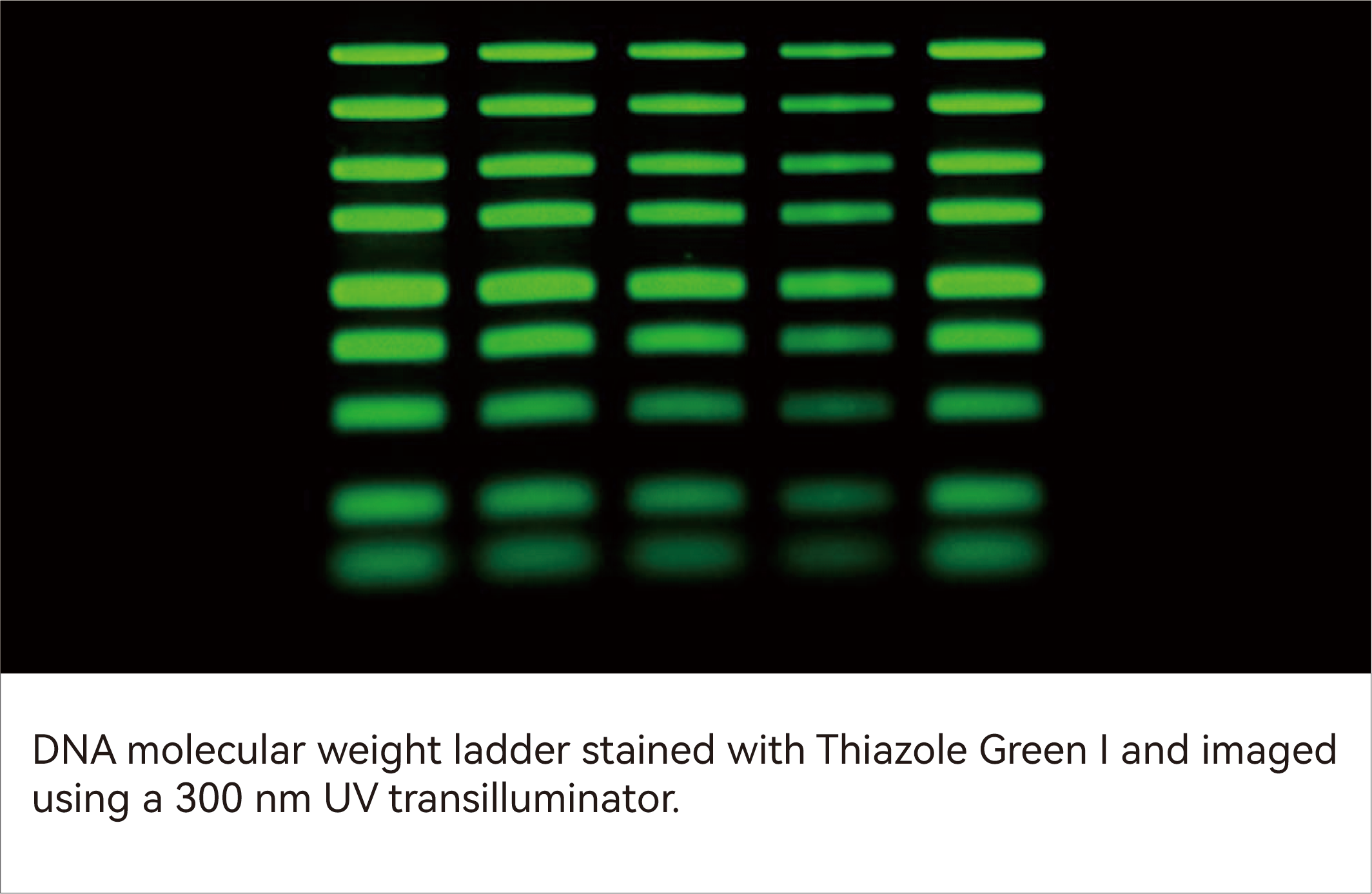
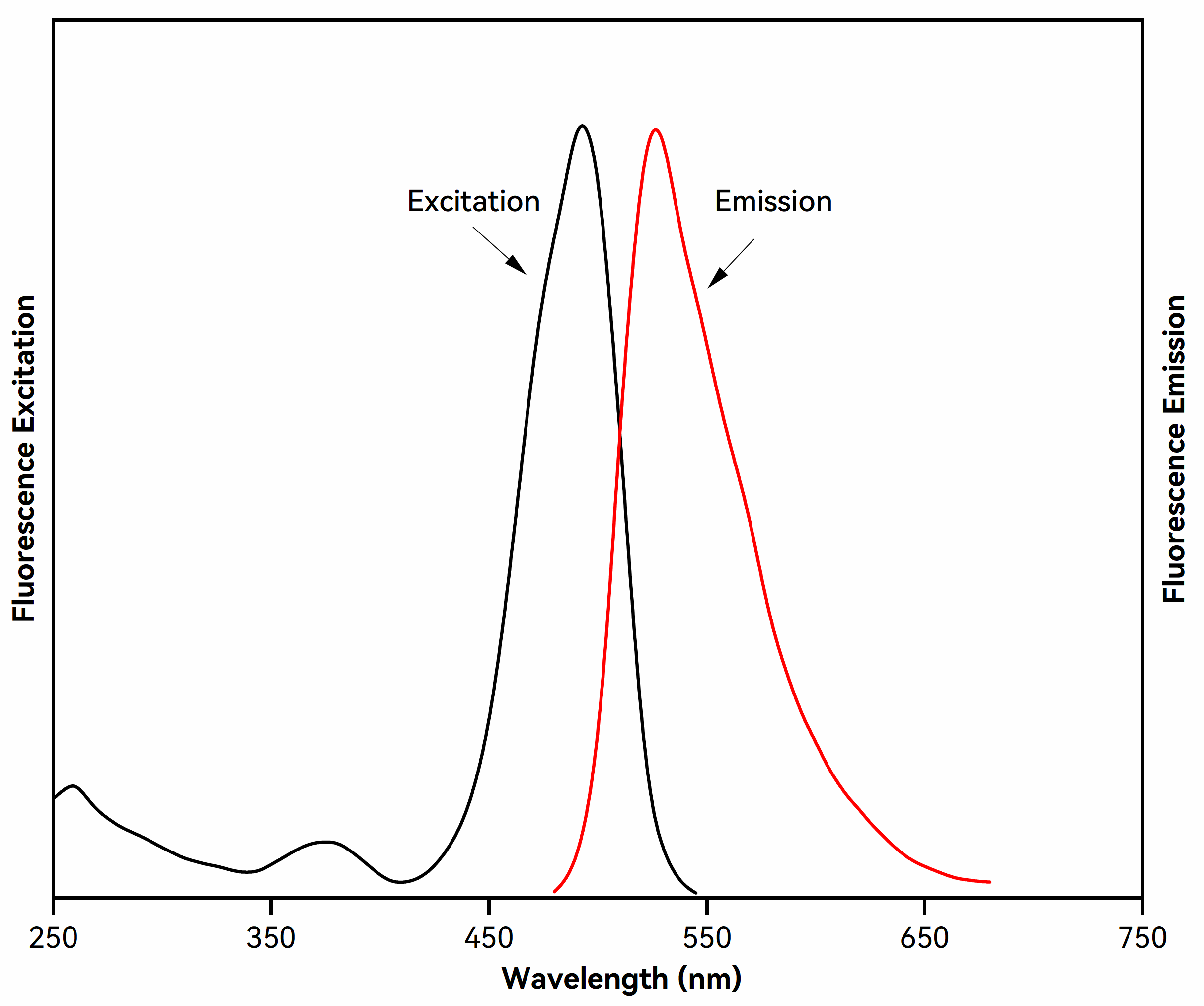


| Issue | Solution |
|---|---|
| Smearing or trailing of DNA bands in pre-stained gels | 1. Reduce the DNA loading amount to 1/2 or 1/3 of the original. Thiazole Green I is significantly more sensitive than ethidium bromide (EtBr), and overloading may cause overexposure or smearing of bands. |
| 2. Consider switching to post-staining instead of pre-staining. Post-staining minimizes interference with DNA migration and improves imaging quality. | |
| 3. For high molecular weight DNA, use a lower percentage agarose gel. Smearing is more common with large DNA fragments. | |
| 4. Use TBE buffer instead of TAE. TBE provides stronger buffering capacity and is better suited for high-resolution electrophoresis. | |
| 5. Avoid using loading buffers containing SDS, as SDS can disrupt dye–DNA binding and alter migration patterns. If SDS is required, post-staining is strongly recommended for optimal results. | |
| Altered DNA migration in pre-stained gels | 1. Reduce the DNA loading amount to 1/2 or 1/3 of the original. |
| 2. Lower the dye concentration; a 0.5× working concentration is recommended for in-gel staining. | |
| 3. Use post-staining instead of pre-staining to minimize interference with DNA migration and improve band resolution and imaging quality. | |
| Reduced fluorescence intensity | 1. The dye may have precipitated. Warm the solution at 40–50 °C for 1–2 minutes and vortex thoroughly to redissolve. |
| 2. Store the dye at room temperature and protect it from light to prevent precipitation caused by low temperatures. |
